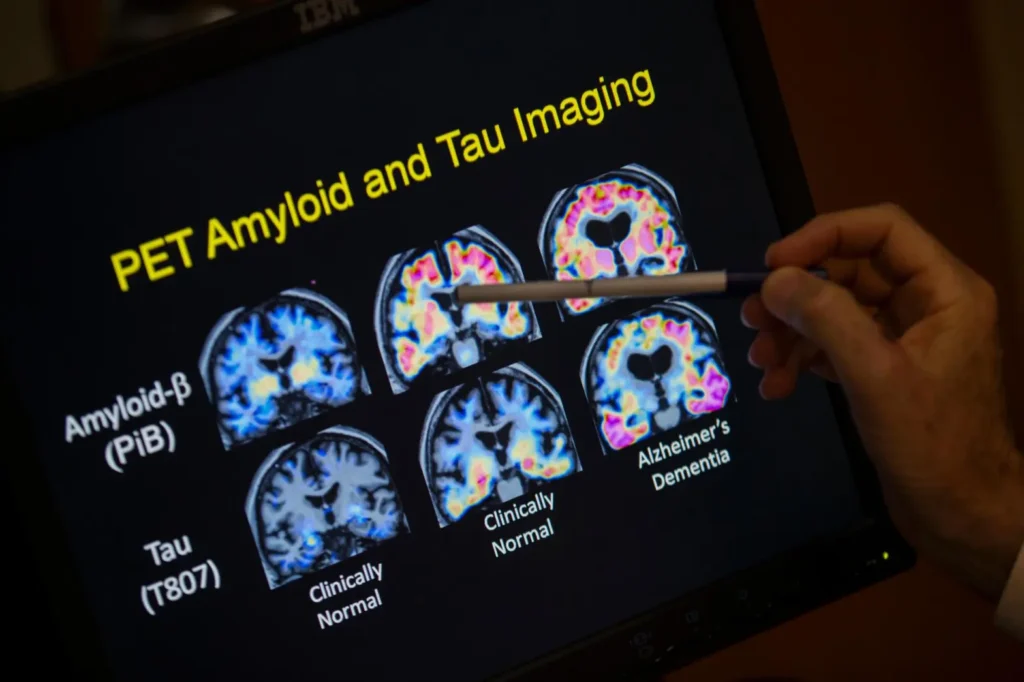
Previously, the only FDA-approved methods for detecting amyloid were invasive tests of spinal fluid or expensive PET scans.
By MATTHEW PERRONE
WASHINGTON (AP) — U.S. health officials on Friday endorsed the first blood test that can help diagnose Alzheimer’s and identify patients who may benefit from drugs that can modestly slow the memory-destroying disease.
The test can aid doctors in determining whether a patient’s memory problems are due to Alzheimer’s or a number of other medical conditions that can cause cognitive difficulties. The Food and Drug Administration cleared it for patients 55 and older who are showing early signs of the disease.
More than 6 million people in the United States and millions more around the world have Alzheimer’s, the most common form of dementia.
The new test, from Fujirebio Diagnostics, Inc., identifies a sticky brain plaque, known as beta-amyloid, that is a key marker for Alzheimer’s. Previously, the only FDA-approved methods for detecting amyloid were invasive tests of spinal fluid or expensive PET scans.
For the full story, please visit bostonherald.com